To Fight Free Radicals: Brain Support Cells To Transfer Their Mitochondria
Nikki Attkisson | Last Updated : August 17, 2022According to recent research published in the Journal of Neuroscience, healthy mitochondria are responsible for stimulating the production of an enzyme that combats free radicals.
A rupture occurs in an artery deep into the mind. When blood rushes into a tissue, it creates free radicals, which in turn cause even more damage.
The bleeding causes damage to the mitochondria, which are the sites in cells responsible for producing energy. After a hemorrhage, astrocytes will transfer the mitochondria they contain to damaged neurons. These healthy mitochondria contain a “therapeutic” peptide called humanin and an enzyme called manganese superoxide dismutase (Mn-SOD) that helps neutralize free radicals. These components work together to protect the cell from damage caused by free radicals.
Demonstrations Regarding The Neurons
Stage of manganese superoxide dismutase (Mn-SOD) in neurons 21 days after bleeding in mice that acquired mitochondria with or without manganese superoxide dismutase at one hour, seven days, and 14 days post-hemorrhage. The treatment caused an increase in the amount of Mn-SOD (shown in purple) in the neurons, as indicated by the cells indicated by the white arrows. Credit goes to Tashiro et al., Journal of Neuroscience Research 2022
After a bleed, the mice were given healthy mitochondria by injection by Tashiro and colleagues. The bleeding caused a decrease in the amount of Mn-SOD found in the brain of the mice and led to an increase in the number of free radicals.
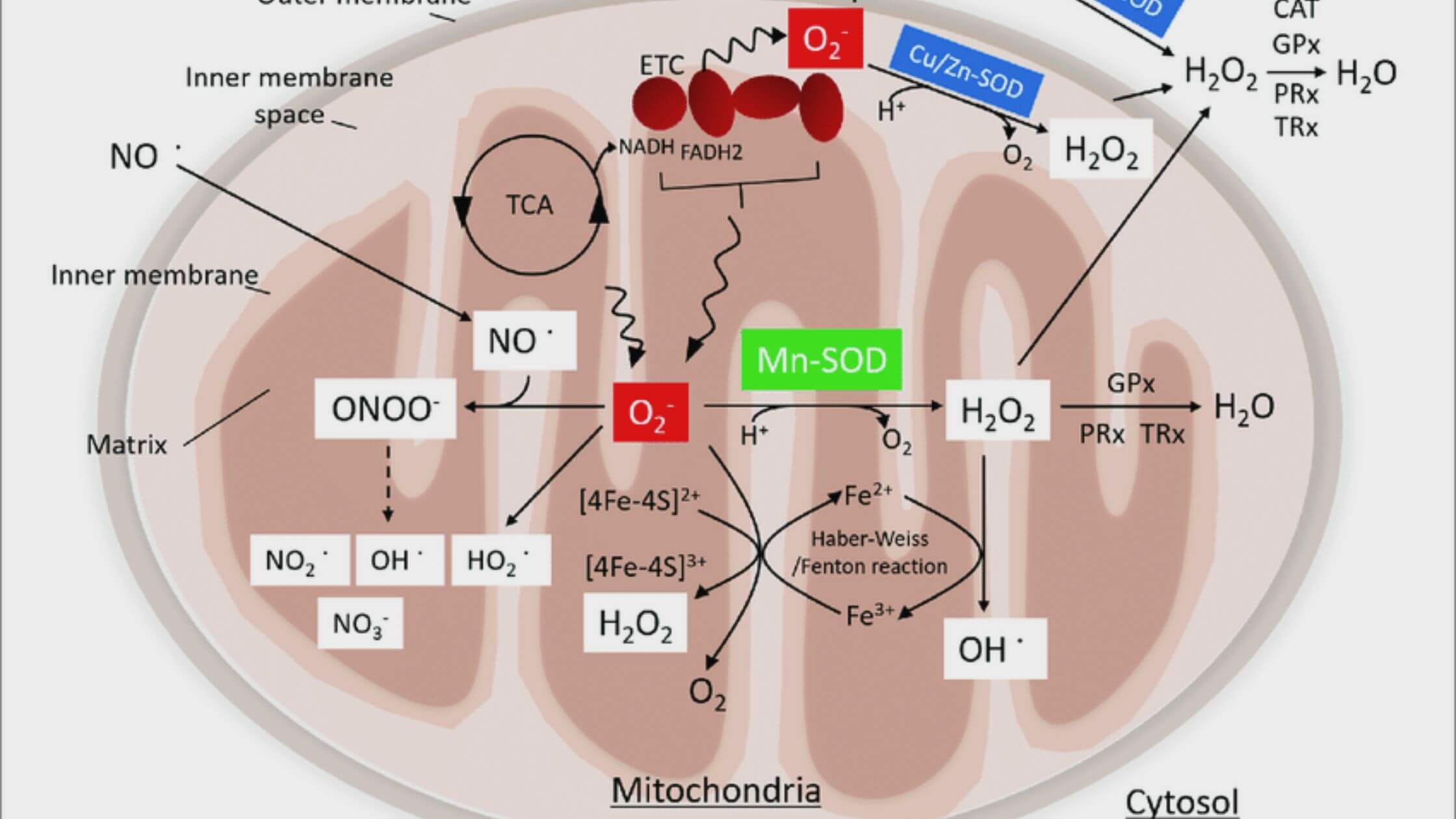
After an injury to the brain caused by intracerebral bleeding, the transplantation of astrocytic mitochondria can adjust the antioxidant protection and neuroplasticity of the neurons and facilitate purposeful healing.
Upon entering neighboring cells, astrocytes release functional mitochondria, also known as Mt, which can regulate processes and promote survival. We recently established that these released Mt might penetrate microglia to promote their reparative/pro-phagocytic phenotype, which aids in hematoma cleanup and neurological repair following intracerebral hemorrhage. [Citation needed] (ICH).
However, it is unknown whether or if the conversion of astrocytic Mt cells into neurons plays a significant role in the defensive processes of the brain after ICH. Here, we found that ICH produces a considerable increase in the production of superoxide radicals and an increase in the extent of oxidative damage, both of which coincide with a deficiency in the mitochondrial enzyme manganese superoxide dismutase (Mn-SOD).
Intravenous transplantation of astrocytic Mt was able to repair the detrimental effects of ICH by restoring Mn-SOD levels and reducing neurological impairments in male mice treated with ICH. This was accomplished by delivering the Mt cells to the brain via the bloodstream. Astrocytic Mt, upon entering neurons, prevented reactive oxygen species-induced oxidative stress and neuronal loss of life by restoring neuronal Mn-SOD levels. At the same time, astrocytic Mt promoted neurite extension and upregulation of synaptogenesis-related gene expression. This was demonstrated utilizing an in vitro ICH-like damage model in cultured neurons.
More From Powdersville Post:
🔵Stress Has The Potential To Strengthen Your Brain! Study
🔵Blood Test Makes It Easy To Identify Mitochondrial Disorders
Findings of the study
In addition, we found that the Mt genome-encoded short peptide humanin (HN), which is typically abundant in Mt, can imitate the Mt-transfer effect on neuronal Mn-SOD production, oxidative stress, and neuroplasticity under conditions similar to those caused by ICH.
This study reveals that adoptive astrocytic Mt switch increases neuronal Mn-SOD-mediated anti-oxidative defence and neuroplasticity inside the brain, both potentiate intentional regeneration following ICH.
Through the use of molecular tags, the researchers could determine that the mitochondria were being taken up by the neurons of the rats from the bloodstream.
The mice given the treatment showed signs of enhanced neurological repair; however, the benefits were lessened when the mice were given mitochondria that lacked the Mn-SOD enzyme. These findings indicate that mitochondria can move across different cells in the brain to promote better health and aid in the healing process.
References:
🔵National Library Of Medicine(n.d)Free radicals, antioxidants and functional foods: Impact on human health(Available Online)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3249911/
🔵National Library Of Medicine(n.d)The Oxygen Free Radicals Originating from Mitochondrial Complex I Contribute to Oxidative Brain Injury Following Hypoxia–Ischemia in Neonatal Mice(Available Online)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3296485/
With over 15 years as a practicing journalist, Nikki Attkisson found herself at Powdersville Post now after working at several other publications. She is an award-winning journalist with an entrepreneurial spirit and worked as a journalist covering technology, innovation, environmental issues, politics, health etc. Nikki Attkisson has also worked on product development, content strategy, and editorial management for numerous media companies. She began her career at local news stations and worked as a reporter in national newspapers.
