FDA Asking To Issue Warnings For Breast Implants
Nikki Attkisson | Last Updated : November 4, 2021Breast implants are a common form of cosmetic surgery which is done on women who have smaller or disproportionate breasts. This is done to make their breasts bigger and make them appear more attractive.
This is a type of cosmetic surgery and is preferred by women in order to boost their self-esteem as well as their sex lives. There are many women who go for breast implants after a mastectomy or even pregnancy.
FDA Asking To Issue Warnings For Breast Implants
Plastic surgeons and psychiatrists are of the opinion that breast i9mplants are a personal decision that women should take after careful thought and should not be done just for pleasing someone or appearing more attractive to them.
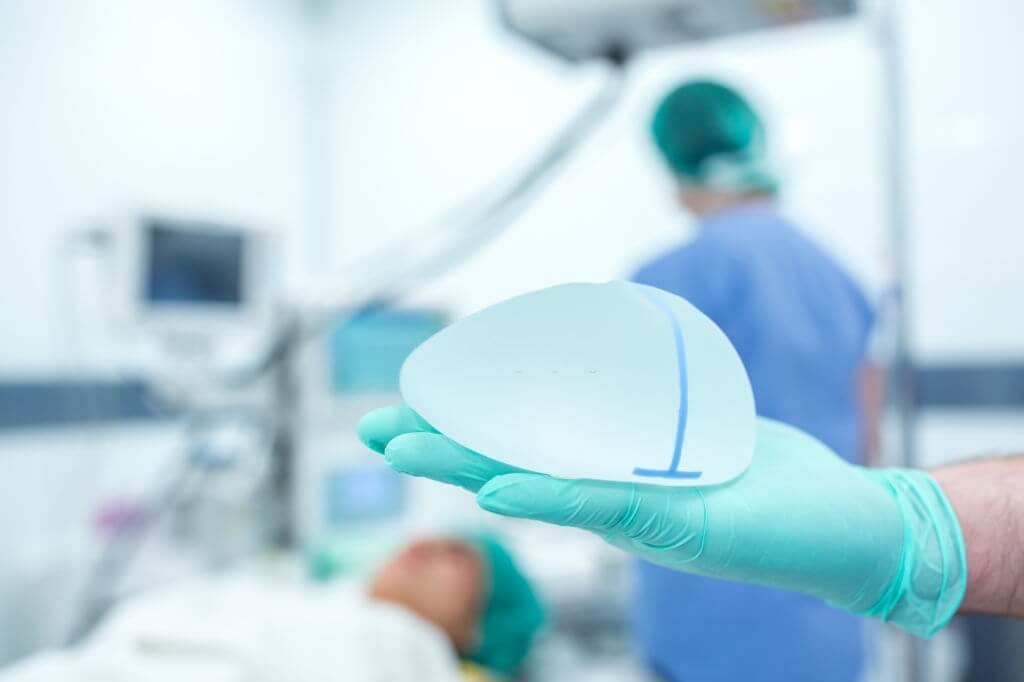
Breast implants are done by means of breast augmentation surgery. This is done by injecting implants of silicone or sterile saltwater solution behind each breast. This solution is usually injected into the crease behind each breast or around the areola. The implant is put between the breast and the chest wall. Placing the implant behind the muscles of the breast gives the implanted breasts a natural look.
There are4, however, some risks to implant surgery and they include infection, rupture, breast implant illness, capsular contracture, and large cell lymphoma. Anaplastic large cell lymphoma occurs among women with implants but implant illness is more common. The common symptoms are chronic fatigue, joint pain, and cognitive difficulties. Dr. Constance Cheng, a breast reconstruction specialist and a plastic surgeon, says that not everyone in the medical community accepts breast implant illness.
The Food and Drug Administration (FDA) has taken cognizance of the risks associated with breast implants and has asked all plastic surgeons to communicate the risks associated with breast implants and also for the material used in the surgery to also have boxed warnings.
The FDA has asked plastic surgeons conducting breast implants to have a complete conversation with women coming for implants and explain all details to them. Such women interesting in getting breast implants will also be required to discuss their intention with their regular healthcare professionals.
Dr. Andy Wongworawat, another certified plastic surgeon, says that patients opting for breast implants must never forget that breast augmentation is actually surgery. Dr. Andy expressed surprise that a stronger warning had not been issued and added that plastic surgeons were already discussing all aspects of breast augmentation surgery with women interested in getting breast implants.
The essence of the warning is that the solution used for implants should only be sold to certified plastic surgeons and that the plastic surgeons using the material explain all aspects including side effects to the women opting for breast implants.
Another certified plastic surgeon, Dr. Alexander Zurlarrain, expressed an opinion that the warning issued by the FDA was directed at patients with the objective of improving their education about breast implants and the possible complications. Other certified plastic surgeons feel that this move will cover up practices of less vigilant checks and ensure that only certified breast implant plastic surgeons conduct this surgery and reduce chances of surgery complications.
The move from FDA has come after women who had undergone breast implants had begun suffering from rheumatoid arthritis, scleroderma, and Sjogren’s syndrome. While there is no clear evidence that these diseases are directly linked with breast implants, recent studies have shown that women with breast implants have a tendency to develop autoimmune systems, and compromised immunity due to the surgery puts the patient at risk of contracting other diseases.
The FDA regulation outlining the risks involved with a deceptively harmless cosmetic surgery could not have come at a better time.
With over 15 years as a practicing journalist, Nikki Attkisson found herself at Powdersville Post now after working at several other publications. She is an award-winning journalist with an entrepreneurial spirit and worked as a journalist covering technology, innovation, environmental issues, politics, health etc. Nikki Attkisson has also worked on product development, content strategy, and editorial management for numerous media companies. She began her career at local news stations and worked as a reporter in national newspapers.
