FDA Hold Up The Authorization Of Covid Vaccine For Younger Children!
Nikki Attkisson | Last Updated : February 17, 2022With an increasing number of cases in America, only the children of 6 months to 5 years had not been vaccinated. Earlier, much data showed that the Omicron variant is affecting younger children more. The need to be vaccinated has risen and companies are working out to vaccinate younger children.
FDA Hold Up The Authorization Of Covid Vaccine For Younger Children
Parents are very eager to get their children vaccinated soon and they think that it is something better than nothing. The Pfizer company has sought out the FDA for authorization for the use of vaccines for younger children.
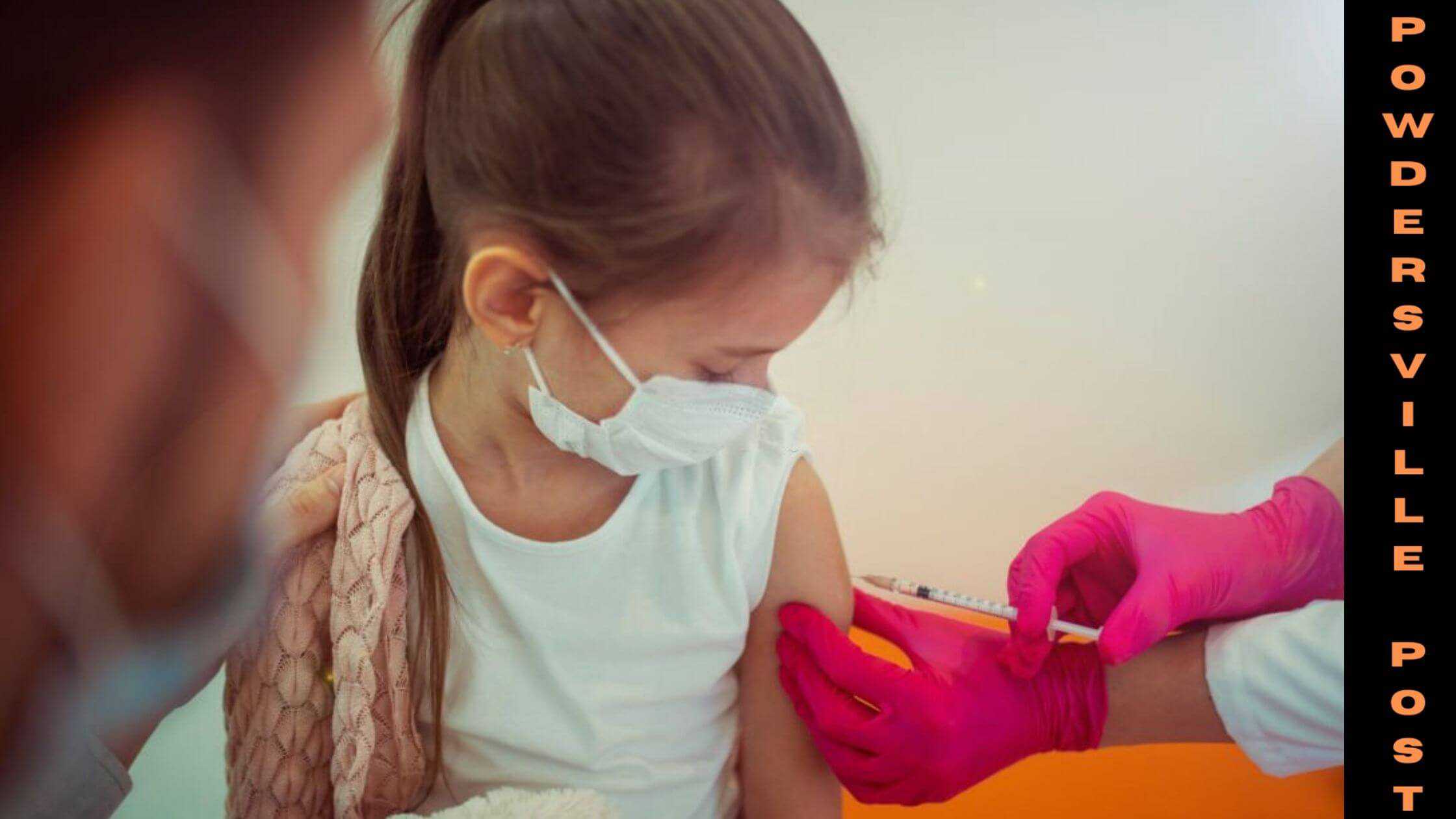
The meeting was scheduled on Friday to discuss vaccinating young children and the pros and cons of it. But after going through the data FDA is not satisfied with the analysis of data and is asking for more information on vaccinating younger children.
So, the meeting was postponed to Tuesday, and the FDA and the companies will discuss and analyze the data and safety of vaccinating 6 months and older children. Pfizer decided first to go with the two doses data, but they are also working on collecting data for the third dose.
Two doses could not provide the needed protection when there is present and future variant in the picture and so they decided to go with the third dose. Pfizer asked for permission to vaccinate with two doses, but the FDA strictly said that they needed more data to come up with a decision. FDA also added that they can submit the data for three doses once altogether.
Many of them criticized the FDA for delaying the authorization, but they said that we have not approved on Tuesday. So, we are taking our time adjusting to this and are not rushing out without analyzing the data.
Many people do feel that FDA delaying during the pandemic situation is not settled well and parents are always ready to grab the opportunity to vaccinate their children.
FDA thinks that when we come up with vaccinating children, then we should be able to assure the parents about the efficacy and protection level of the vaccines. They also assured that they will do their best in every way and move forward with this as quickly as they have the needed data and they advised the parents to go on with the safety measures for their children till then.
Pfizer said that they are expecting the data to be completed for three doses by April. Pfizer’s early data stated that two doses will not provide the necessary immune protection, so they decided to go with third dose clinical trials.
Key background
Younger children are still not vaccinated and the need for it is raised now because the new variant is affecting more people. song withThe risk of being highly infected and hospitalized has increased now and only vaccines can reduce the risk of being hospitalized. So, the companies and government decided to vaccinate children of 6 months to 5 years. But enough data is not provided to go ahead with the third dose trial still going on.
Some parents fear that the children cannot take up the pain and power of the vaccine. Some children can also experience side effects and some are already infected with some other diseases.
The FDA is carefully handling these subjects because it involves the risk of children and takes time to analyze the potency and safety of getting vaccinated. The data also seems insufficient and with clinical trials going on, it is not possible to come up with a decision soon. They have also said that it is not considered as any but not right now.
With over 15 years as a practicing journalist, Nikki Attkisson found herself at Powdersville Post now after working at several other publications. She is an award-winning journalist with an entrepreneurial spirit and worked as a journalist covering technology, innovation, environmental issues, politics, health etc. Nikki Attkisson has also worked on product development, content strategy, and editorial management for numerous media companies. She began her career at local news stations and worked as a reporter in national newspapers.
