A Study Has Proven That Mixing And Matching Corona Virus Booster
Nikki Attkisson | Last Updated : October 15, 2021As the United States Food and Drug Administration has been contemplating the possibility of booster vaccine shots for Johnson & Johnson and Moderna recipients, comes the positive feedback from a highly anticipated study that mixing and matching COVID 19 booster doses can prove to be effective and safe.
Mixing and matching refer to administering a booster shot of a vaccine that is different from the vaccine that is used for the initial vaccination regime.
A Study Has Proven That Mixing And Matching Corona Virus Booster
As per the initial data from a National Institutes of Health study, it has been concluded that all the combinations of mixing and matching Coronavirus booster vaccine shots do increase the body’s immune response without compromising on the safety bit.
This research is likely to be submitted to the advisory committee
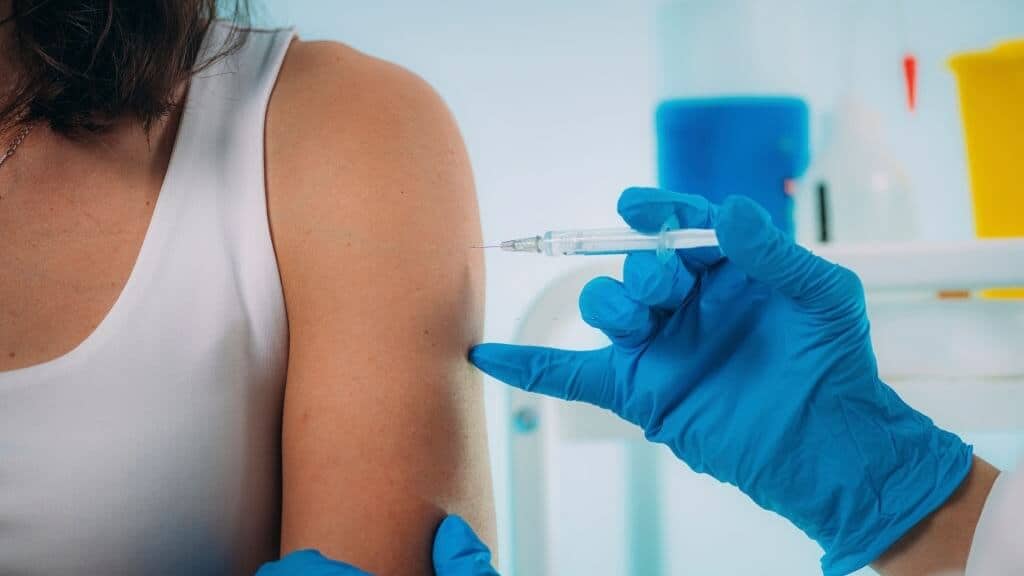
Of the Food and Drug Administration on Friday, 15th October 2021.
This mix and match formula seemed to be the most effective for the single-dose Johnson & Johnson vaccine recipients, who need to get their second shot from either Pfizer or Moderna.
The Heterologous vaccine study, which is yet to be evaluated by peers, concluded that all those who were originally given the Moderna and Pfizer vaccines and received either of the drugmaker’s booster shots had a more or less similar immune response.
However, the researchers who conducted the study clarified that this study was not meant to draw a direct comparison between responses of different booster regimens.
The study results come at a time when the American Food and Drug Administration has been pondering over what to do about booster doses for Johnson & Johnson and Moderna recipients.
The study was sponsored by the Infectious Diseases Clinical Research Consortium (IDCRC) through the National Institute for Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), with due support from the National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Influenza Vaccine Innovation Centers (CIVICs) as well as NIH Vaccine Research Center.
As further progress, the U.S Food and Drug Administration committee is scheduled to have a meeting on Thursday, 14th October 2021 in order to further discuss the booster vaccine shots for Moderna recipients and another meeting has been scheduled on Friday, 15th October 2021 to discuss the possibility of another Johnson & Johnson vaccine dose. Mixing and matching of booster vaccine shots will also be discussed by the experts at the meetings.
The data that was released by the U.S Food and Drug Administration on Wednesday,13th October 2021 reported a few limitations in the booster data for Johnson & Johnson vaccine recipients, which has possibly set up the FDA committee meeting on Friday to discuss multiple questions.
While all health officials claim that all the COVID 19 vaccines still prove to be effective in providing high levels of protection against Coronavirus, there is still some concern that the protection against mild cases of infection could possibly be waning with time.
With over 15 years as a practicing journalist, Nikki Attkisson found herself at Powdersville Post now after working at several other publications. She is an award-winning journalist with an entrepreneurial spirit and worked as a journalist covering technology, innovation, environmental issues, politics, health etc. Nikki Attkisson has also worked on product development, content strategy, and editorial management for numerous media companies. She began her career at local news stations and worked as a reporter in national newspapers.
