Adolescents Should Receive The Novavax Vaccine Between 12 Years?
Nikki Attkisson | Last Updated : August 29, 2022The Australian technical advisory group on immunization issued recommendations on using the Novavax vaccine last week. This recommendation has now allowed vaccination of adolescents between the ages of 12 to 17 years. This vaccine has been approved by Australia’s public health department and goods administration. It has evaluated the data on the efficacy and safety of this vaccination for adolescence in this age group.
It has been discovered that the use of this vaccine in adolescents is in the backdrop of International recommendations approved by organizations like the World Health Organisation.
This acceptance is provided to prevent the infection in school-going children from covid 19. The government has decided to initiate a vaccination program for the vaccination of adolescents between the age group of 12 to 17 years, and this program will commence on 5th September 2022.
Recommendations
According to the recommendations issued by this research body, Three Types of vaccination can be used in this age group. The first one is Nova wax, Pfizer, and Moderna. But out of all three, it is the first kind of vaccine that would be the safest and the most effective in the children in this group. The group has also identified the number of doses and the time gap in which both must be administered to adolescents.
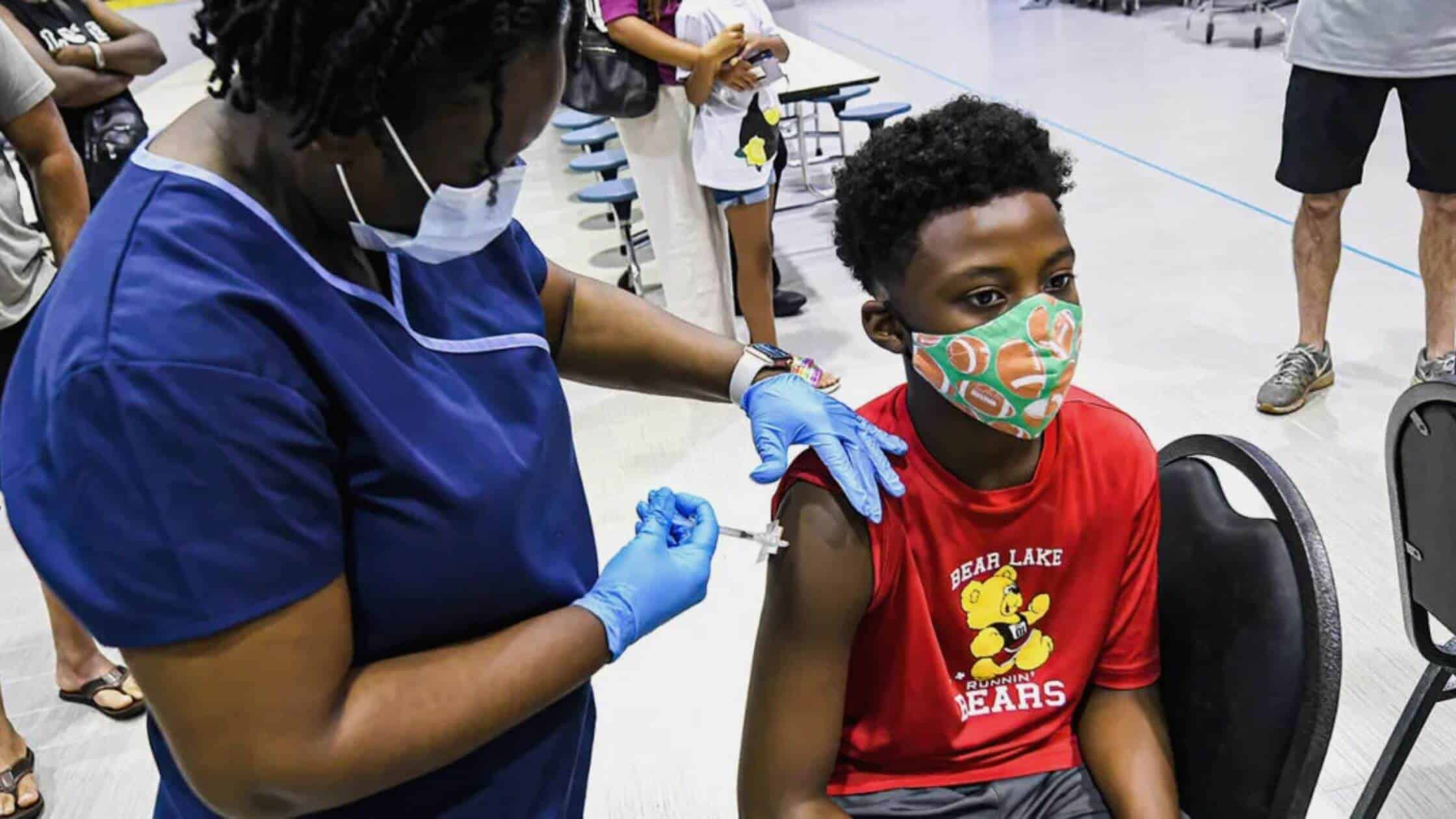
There must be two doses of this vaccination; not only this, but the duration between the two doses must be two weeks apart which can be extended to around 8 weeks. The amount of time gap between both doses can also be extended because it would be responsible for increasing the efficiency and effectiveness of the treatment. It is also advised to control the health and dietary habits around vaccination to reduce the risk of myocarditis and pericarditis. It has also advised people with severe immunocompromise to receive around 3 doses of the vaccine.
Booster doses
Presently, no booster dose of this kind of vaccine is registered with TGA, especially for adolescents aged 12 to 17 years. But the research institution has advised the company to develop a booster dose as soon as possible so that the booster dose also gets administered to the body in every situation. The institute has also said that this booster dose is helpful for insurance of the efficiency of the treatment.
It becomes essential to understand that if the company producing this vaccine does not produce the booster dose, then the booster dose of some other brand would be recommended by the institute for adolescents. The government will soon be preparing the list of the associated document, which must be submitted for the company’s approval of the booster dose.
More From Powdersville Post:
🔵Novavax Emergency Covid-19 Vaccine Awaiting FDA Nod
🔵A Future Vaccine That Will Stop the Growth of the Pandemic Finally
Actions required
The government will soon be opening an online portal for the registration of adolescents who will be seeking vaccination from 5th September onwards. The government will also issue a list of the documents the adolescents must submit at the time of vaccination. All the rules and regulations in this regard must be strictly complied with.
Conclusion
It can be ultimately concluded that this is a practical step to prevent the spread of coronavirus in adolescents. They have to be protected against the infection in every situation because this infection leaves a very detrimental impact on their health. It is advised to get the doses on time so that the effect of the vaccine on the human body can be discovered in a short interval. It is also important because the students are at a higher risk of infection in Australia.
References:
🔵Centers For Disease Control And Prevention (n.d) Novavax COVID-19, Adjuvanted Vaccine: Overview and Safety (Available Online):https://www.cdc.gov/coronavirus/2019-ncov/vaccines/novavax.html
🔵FDA (n.d) Coronavirus (COVID-19) Update: FDA Authorizes Emergency Use of Novavax COVID-19 Vaccine, Adjuvanted (Available Online):https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-emergency-use-novavax-covid-19-vaccine-adjuvanted
With over 15 years as a practicing journalist, Nikki Attkisson found herself at Powdersville Post now after working at several other publications. She is an award-winning journalist with an entrepreneurial spirit and worked as a journalist covering technology, innovation, environmental issues, politics, health etc. Nikki Attkisson has also worked on product development, content strategy, and editorial management for numerous media companies. She began her career at local news stations and worked as a reporter in national newspapers.
